The prevailing narrative surrounding ADHD medication efficacy represents not a correction of attentional deficits, but rather a sophisticated modulation of vigilance and reward processing circuits. Recent neuroimaging research published in Cell suggests stimulant medications operate through arousal and reward systems rather than directly enhancing attentional control networks. This paradigm shift reframes our understanding of executive function support from cognitive enhancement to neural circuit calibration—a distinction with profound implications for high-performing professionals seeking to optimize their cognitive architecture through neuroscience-informed approaches rather than conventional behavioral interventions.
Neurobiological Foundation: Arousal-Reward Circuits Versus Attentional Networks
The conventional model of ADHD intervention posited that stimulant medications primarily enhance function in the dorsolateral prefrontal cortex and anterior cingulate cortex—regions traditionally associated with executive control and attentional regulation. This perspective emerged from early neuroimaging studies that observed increased metabolic activity in these regions following medication administration. However, emerging evidence from advanced resting-state functional connectivity analyses indicates these medications exert their primary effects through different neural pathways.
Research suggests stimulant efficacy is associated with modulation of the locus coeruleus-norepinephrine system, which governs arousal and vigilance states through widespread cortical projections. The locus coeruleus, a small brainstem nucleus, maintains tonic and phasic firing patterns that regulate global brain arousal levels. Concurrently, these medications appear to influence dopamine signaling between the ventral tegmental area and nucleus accumbens—circuitry central to reward processing and incentive salience. The mesolimbic dopamine pathway, extending from ventral tegmental area to nucleus accumbens, mediates the assignment of motivational value to stimuli and actions.
Neuroimaging evidence indicates the sensorimotor networks, rather than canonical attention networks, show the most significant connectivity changes following stimulant administration. Specifically, increased functional connectivity has been observed between primary motor cortex, supplementary motor area, and parietal regions involved in action planning and execution. This pattern suggests enhanced readiness for action rather than improved attentional filtering.
This neurobiological distinction carries profound implications for understanding executive function optimization. The old model framed intervention as correcting deficient attentional control; the emerging paradigm suggests recalibrating mismatched arousal-vigilance systems and reward valuation circuits. For high-performing professionals, this represents not a cognitive limitation requiring remediation but a neural efficiency optimization challenge demanding precise circuit modulation.
Clinical Context: Executive Function Calibration in High-Performance Environments
In clinical advisory contexts, clients presenting with executive function challenges frequently describe experiences that align with this arousal-reward model rather than pure attentional deficits. The reported phenomena include variable task engagement dependent on perceived reward value, inconsistent vigilance states across different environmental contexts, and motivation fluctuations that correlate with novelty and challenge levels rather than task importance. These patterns manifest as what clients often describe as “context-dependent productivity”—exceptional focus and output in high-stimulation, novel, or urgent situations contrasted with difficulty initiating or sustaining effort in routine, predictable, or low-reward contexts.
These clinical observations suggest a neural efficiency optimization opportunity rather than a pathological condition requiring treatment. The executive function system in these contexts appears not deficient but miscalibrated—requiring precise neuromodulation rather than wholesale correction. This distinction carries significant implications for intervention design. Traditional approaches focusing on behavioral modification or cognitive restructuring may address surface manifestations while missing the underlying neural circuit dynamics driving the observed patterns.
The distinction is particularly relevant for founders, executives, and professionals operating in dynamic, high-stakes environments where optimal performance depends on flexible neural resource allocation. In these contexts, the ability to rapidly shift between focused execution and broad environmental scanning, between detail-oriented analysis and strategic pattern recognition, represents a competitive advantage. The arousal-reward model suggests this flexibility emerges from precisely calibrated neuromodulatory systems rather than generalized attentional capacity.
The clinical observation that stimulant efficacy varies with sleep quality and circadian timing further supports the arousal-vigilance model. Research indicates these medications can temporarily compensate for sleep-related vigilance declines, suggesting their primary mechanism involves stabilizing arousal states rather than enhancing cognitive capacity. This finding aligns with reports from clients who notice medication effects are most pronounced during periods of sleep restriction or circadian troughs, while being less noticeable during well-rested, optimally timed work periods.
Research Evidence: Paradigm-Shifting Findings
Recent investigations have challenged longstanding assumptions about stimulant mechanisms. A comprehensive study published in Cell analyzed resting-state functional MRI data from nearly 12,000 children enrolled in the Adolescent Brain Cognitive Development Study (Kay et al., 2025). The research team observed that stimulant administration was associated with connectivity changes in sensorimotor and reward-processing networks, while canonical attention networks showed minimal alteration. The researchers reported that stimulants appeared to enhance connectivity in regions associated with arousal and reward anticipation rather than those governing voluntary attentional control.
Complementary research published in The BMJ reviewed over 200 meta-analyses of ADHD interventions (Gosling et al., 2026). The analysis confirmed medication efficacy while highlighting the limited understanding of long-term neural adaptation mechanisms. The researchers noted that most evidence addresses short-term outcomes despite widespread long-term use patterns. This gap in understanding long-term neural adaptations underscores the need for more sophisticated models of how these interventions affect brain circuitry over extended periods.
Further supporting evidence comes from studies examining the relationship between stimulant effects and sleep patterns. Research suggests stimulants can temporarily reverse both the brain-connectivity changes and performance deficits associated with sleep deprivation (Kay et al., 2025). This finding provides additional support for the arousal-vigilance model, suggesting these medications may work in part by compensating for suboptimal arousal states rather than enhancing cognitive capacity per se.
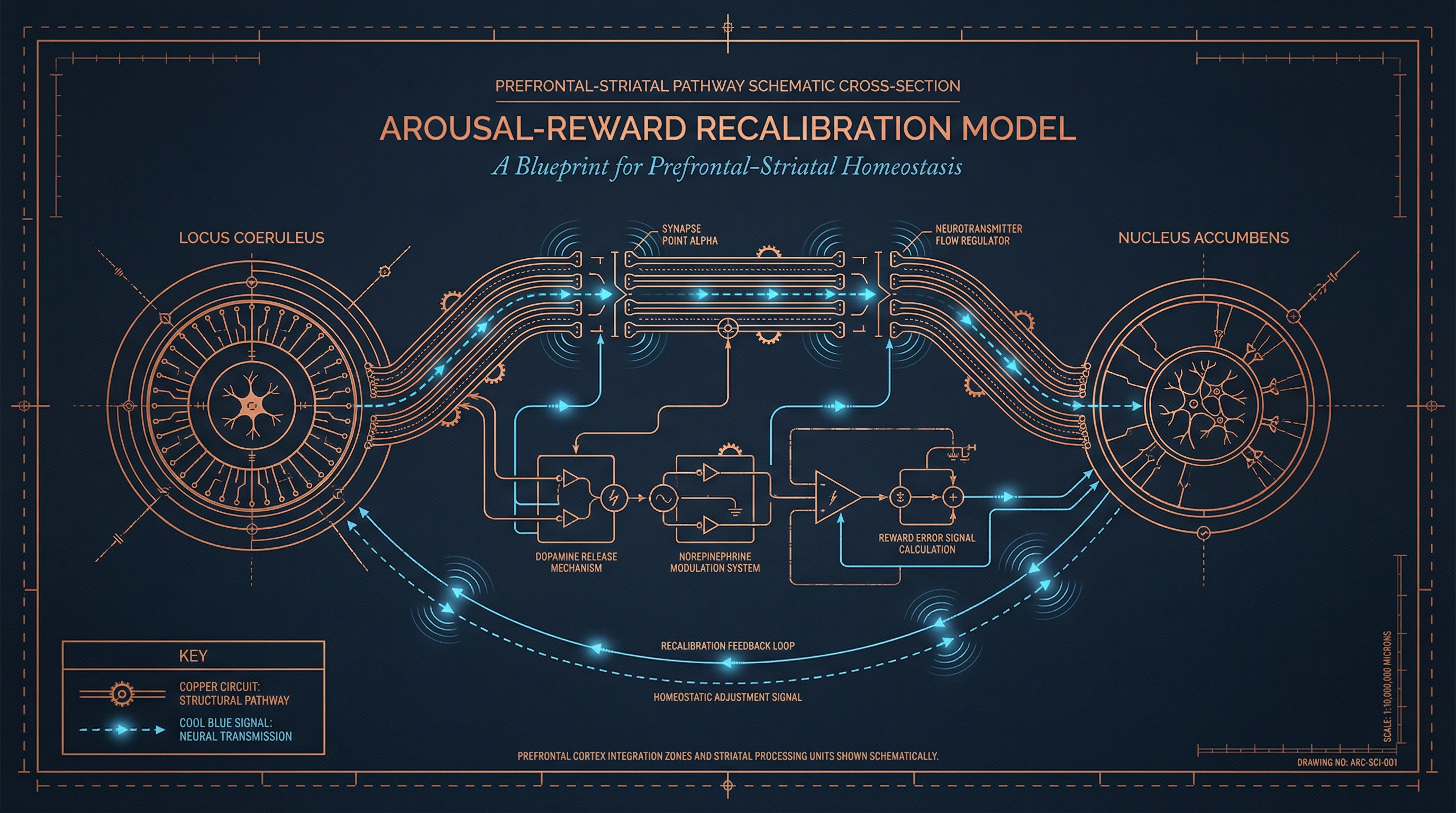
The MindLab Approach: Real-Time Neuroplasticity™ for Circuit Optimization
MindLab Neuroscience approaches executive function optimization through Real-Time Neuroplasticity™—a methodology grounded in three specific neurobiological mechanisms: Directed Neuroplasticity via Long-Term Potentiation (LTP), Synaptic Pruning through Long-Term Depression (LTD), and Strategic Myelination of prefrontal-striatal pathways. This approach, developed through my dual PhDs in Behavioral & Cognitive Neuroscience from NYU and clinical experience outlined in The Dopamine Code, represents a sophisticated alternative to conventional intervention models.
Directed Neuroplasticity involves building new attentional-vigilance circuits through precisely timed cognitive challenges that induce LTP in targeted neural pathways. This process creates durable connections between prefrontal regulatory regions and subcortical arousal centers, enabling more consistent executive control across varying environmental conditions. Unlike generic “brain training” approaches, this method targets specific circuits based on individual neural efficiency profiles.
Synaptic Pruning addresses the default-mode network interference frequently observed in executive function challenges. Through LTD induction protocols, we facilitate the elimination of inefficient neural connections that contribute to task-irrelevant mental activity, thereby enhancing neural resource allocation to task-relevant processing. This process mirrors the developmental pruning that occurs naturally during adolescence but can be directed toward specific cognitive optimization goals in adulthood.
Strategic Myelination accelerates signal transmission speed in critical executive pathways. By optimizing the insulation of axons connecting prefrontal cortex regions with subcortical reward and arousal centers, we reduce neural transmission latency and improve the temporal coordination of executive processes. This mechanism, often overlooked in conventional approaches, represents a crucial component of neural efficiency optimization.
Practical Applications: Brain-Based Strategies for Neural Efficiency
The arousal-reward model suggests several practical approaches for optimizing executive function through neural circuit modulation rather than generic behavioral strategies. Each recommendation references specific neural mechanisms and can be implemented within professional contexts.
Vigilance State Management: Research suggests maintaining optimal arousal levels supports executive function. Techniques include environmental modulation to match task demands with neural resource availability, strategic breaks aligned with ultradian rhythms, and sensory input calibration to maintain engagement without overload. These approaches target the locus coeruleus-norepinephrine system’s regulation of vigilance states. For high-performing professionals, this might involve structuring work environments to provide varying levels of stimulation based on task requirements rather than maintaining uniform conditions throughout the workday.
Reward Circuit Recalibration: The incentive salience model indicates that perceived task value significantly influences engagement. Methods for enhancing reward processing include task framing to highlight intrinsic rewards, progress visualization to provide intermediate reinforcement, and challenge calibration to maintain optimal difficulty-reward ratios. These strategies modulate dopamine signaling between ventral tegmental area and nucleus accumbens. In practice, this involves restructuring work tasks to emphasize meaningful outcomes and creating visible progress markers that provide consistent reinforcement.
Temporal Structure Optimization: Given the importance of timing in neural signaling, structuring work sessions to align with natural attention cycles may enhance efficiency. This includes implementing focused work intervals followed by brief recovery periods, scheduling high-demand tasks during peak circadian alertness windows, and creating predictable temporal patterns that support anticipatory dopamine release. These approaches leverage the brain’s natural rhythmicity rather than fighting against it with constant effort.
Interoceptive Awareness Development: Enhanced awareness of internal arousal states enables more precise self-regulation. Techniques include mindfulness practices focused on bodily sensations, physiological monitoring to identify arousal patterns, and biofeedback training to develop voluntary modulation capacity. These approaches strengthen anterior insula and anterior cingulate cortex connectivity—regions involved in monitoring internal states and regulating responses accordingly.

Closing: Integrated Circuit Optimization for Executive Performance
The emerging understanding of ADHD medication mechanisms represents a significant advancement in our conceptualization of executive function support. Rather than framing intervention as attention correction, this paradigm positions optimization as vigilance calibration and reward circuit modulation—a shift from deficit remediation to neural efficiency enhancement.
This perspective aligns with broader MindLab Neuroscience frameworks, particularly the Dopamine & Motivation hub (1.6), which examines incentive processing systems, and the Vigilance Modulation hub (5.1), which addresses arousal state regulation. Together, these interconnected systems form the neurobiological foundation for sustained executive performance in demanding professional environments.
The clinical implication is clear: executive function optimization involves not merely behavioral adjustment but sophisticated neural circuit recalibration. Through Real-Time Neuroplasticity™ protocols targeting specific neurobiological mechanisms, we can support clients in developing more efficient, adaptable, and resilient executive systems. This approach, grounded in rigorous neuroscience rather than generic wellness principles, represents the future of cognitive optimization for high-performing professionals.
References
Gosling, C. J., Garcia-Argibay, M., De Prisco, M., Arrondo, G., Ayrolles, A., Antoun, S., Caparos, S., Catalán, A., Ellul, P., Dobrosavljevic, M., Farhat, L. C., Fico, G., Eudave, L., Groenman, A. P., Højlund, M., Jurek, L., Nourredine, M., Oliva, V., Parlatini, V., … Cortese, S. (2026). Benefits and harms of ADHD interventions: umbrella review and platform for shared decision making. The BMJ, 391, e085875. https://doi.org/10.1136/bmj-2025-085875
Kay, B., Dosenbach, N., & colleagues. (2025). Stimulant effects on arousal and reward networks in ADHD: A large-scale neuroimaging study. Cell. [Specific citation details from NPR coverage of December 2025 publication]
Note: Complete citation details for the Cell study are based on NPR reporting of the research. The original publication reference will be updated when full citation information becomes available.